The kidneys are among the most commonly affected organ systems in sickle cell anemia (SCA) and an estimated 15% of deaths in patients with SCA are attributed to kidney failure (PMID 7993409). Angiotensin converting enzyme-inhibitors and angiotensin receptor blockers have been adopted to treat SCA-related chronic kidney disease (CKD) from the non-SCA literature, although demonstrated efficacy in SCA CKD has been marginal in a limited number of small, short-term studies (PMID 28589652). Therapies that directly target the pathophysiology of SCA-related kidney disease may stabilize or improve kidney function. Both the rate of hemolysis and the degree of anemia are associated with the development and progression of CKD in SCA (PMID 24329963, 21523807). Voxelotor inhibits hemoglobin polymerization in SCA red blood cells in the deoxygenated state, leading to a reduction in the rate of hemolysis and an increase in hemoglobin concentration.
We conducted a pilot study to determine whether voxelotor can improve kidney function in adults with SCA (NCT 04335721). SCA (HbSS or HbSβ 0-thalassemia) patients ≥ 18 years old and with urine dipstick-defined hemoglobinuria (+ for blood & ≤ 2 red blood cells/high power field), CKD stage 1 or 2 (urine albumin-to-creatinine ratio [ACR] ≥ 30 mg/g & estimated glomerular filtration rate [eGFR] ≥ 60 mL/min/1.73m 2), and hemoglobin concentrations of 5.5 - 10.5 g/dL were eligible. The eGFR was calculated using the CKD-EPI 2009 creatinine-based equation without the race coefficient and the 2012 cystatin C-based equation. Patients were randomized to voxelotor 1,500mg/day for 48 weeks or standard of care, as defined by their primary SCA provider. First or second void urines were collected for urine ACR measurements. The primary endpoint was the change in urine ACR from the mean values at baseline and screening versus the mean values at 47 and 48 week visits. One patient randomized to standard of care was started on voxelotor at week 24 by his provider to improve his hemoglobin concentration. This patient is included in the voxelotor treatment group in combined analysis; the change in laboratory measures in this patient is based upon changes from the mean of values at week 12 and 24 to week 47 and 48 visits. Median and range values are provided.
Between 3/2021 and 6/2022, 8 SCA patients with hemoglobinuria and urine ACR > 30mg/g crt observed during routine clinic visits were screened and 6 met the inclusion criteria. The median age of the enrolled cohort was 36 (27 - 63) years, 4 were male, 4 were on stable doses of hydroxyurea (median dose 1000mg/day; 500 - 1500mg/day) and 2 were on stable doses of lisinopril (5, 20mg/day) for at least 90 days before screening. Two other patients had been on lisinopril in the past but developed hyperkalemia prior to enrollment. The median values of kidney function measures at screening and baseline study visits were as follows: urine ACR, 251 (32 - 3132) and 392 (80 - 3101) mg/g crt; creatinine-based eGFR, 109 (93 - 136) and 115 (88 - 137) mL/min/1.73m 2; and cystatin C-based eGFR, 82 (61 - 141) and 85 (61 - 128) mL/min/1.73m 2, respectively.
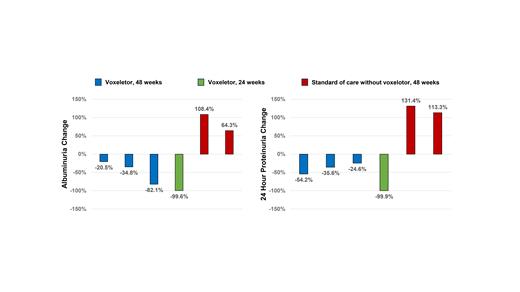
SCA patients who received voxelotor (n = 4) had a median increase in hemoglobin concentration of +0.9 g/dL (-0.4 - +2.6 g/dL) and reductions in indirect bilirubin concentration of -2.2 mg/dL (0 - -4 mg/dL) and reticulocyte % of -2.7% (-1.2 - -4.9%). Urine ACR (-59%, -21% - -100%) improved in SCA patients treated with voxelotor but worsened in those who received standard of care without voxelotor (n = 2) (+86.3%, 64 - 108%) (P=0.009) (Figure 1A). 24-hour urine protein concentrations also improved in those that received voxelotor (-45%, -25% - -100%) but increased in those who did not receive voxelotor (+122%, 113 - 131%) (P = 0.002) (Figure 1B). Changes in creatinine- and cystatin-C based eGFR from baseline were not significantly different between patients who received voxelotor and those who did not.
This pilot study demonstrates that SCA patients with hemoglobinuria and early stages of CKD treated with voxelotor may have significant improvements in urine ACR and proteinuria after 24 - 48 weeks of therapy. These preliminary results support the concept that improving hemolytic anemia could be a targeted therapeutic approach to reduce biomarkers of kidney dysfunction, and they warrant larger, prospective clinical studies.
OffLabel Disclosure:
Saraf:Forma Therapeutics: Consultancy, Other: Advisory board, Research Funding; BEAM Therapeutics: Consultancy, Other: Advisory board; Agios: Consultancy, Other: Advisory board; GBT/Pfizer: Consultancy, Other: Advisory board, Research Funding, Speakers Bureau; Novartis: Consultancy, Other: Advisory board, Research Funding. Gordeuk:GBT/Pfizer: Consultancy, Research Funding; Novartis: Research Funding; Incyte: Research Funding; Emmaus: Consultancy, Research Funding; Forma: Consultancy, Research Funding; CSL-Behring: Consultancy; Modus Therapeutics: Consultancy; Takeda: Consultancy.
Voxelotor reduces hemolytic anemia in sickle cell anemia. This trial investigates whether voxelotor stabilizes or improves kidney function in patients with sickle cell anemia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal